Abstract
Background/aims Cardiovascular disease (CVD) is a leading cause of death in lower-risk Myelodysplastic syndrome (LR-MDS) patients and a shared pathobiology between MDS and CVD has been postulated. However, current evidence emanates only from retrospective cohorts suffering from crucial shortcomings. Prospective annotation can overcome these limitations and delineate the role of MDS as an independent risk factor for CVD. For this reason we conducted a prospective observational single-centre cohort study in LR-MDS patients.
Methods Patients underwent thorough evaluation for CVD every 6 months. Cerebrovascular, peripheral and coronary vascular beds were assessed for atherosclerosis by ultrasound and coronary artery calcium (CAC), respectively. Cardiac structure and function was assessed by echocardiography. Multi-Ethnic Study of Atherosclerosis (MESA), Framingham (FRS) risk scores and serum markers of myocardial injury (Serum high-sensitivity troponin T, hsTnT), stress (N-terminal pro-B-type natriuretic peptide, NT-proBNP), and systemic inflammation (high-sensitivity C-reactive protein, hsCRP) were also estimated at each visit. Chi-square, Wilcoxon signed-ranks tests and Pearson's correlation coefficient were used as appropriate. Multiple linear regression was applied for modeling the relationship between a dependent and one or more independent variables. The level of statistical significance was set to P=0.05.
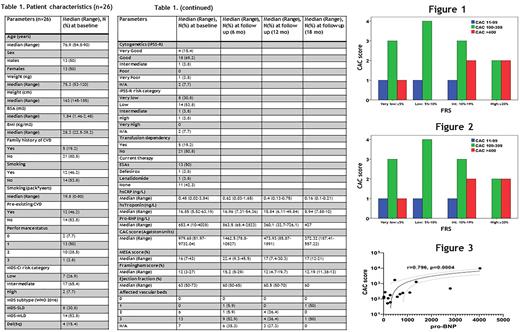
Results Table 1 presents baseline characteristics of 26 patients included so far. There were no correlations of CAC, MESA and FRS with either MDS subtype or IPSS-R risk category after adjustment for preexisting CVD, age, sex, and transfusion dependence. In sharp contrast to the general population (Okwuosa, TM et al. JACC 2011), CAC score was not associated with FRS (p=0.7) and was disproportionately increased in low and very low FRS risk patients (Figure 1). Likewise, the number of affected vascular beds (AVB) did not correlate with FRS (p=0,51) (Figure 2), further suggesting that the current CVD risk prediction tools are inaccurate in MDS and arguing for an intrinsic tendency of MDS towards CVD development.
A second and a third evaluation was available in 17 and 7 patients, respectively. All patients increased significantly the CAC score both at 6 months (p=0.04) and 1 year (p=0.003). The average increase from baseline was 26.2% at 6 months and 72.6% at 1 year, markedly higher than expected in the general population (McCullough PA et al. Arch Int Med 2009), and was marginally higher in transfusion dependent patients (p=0.049), but not associated with baseline MESA score, FRS, preexisting CVD, exposure to ESAS, statin therapy, sex. Finally, baseline NT-proBNP levels correlated strongly with CAC score P=0.001 (Figure 3) in line with numerous evidence showing that NT-proBNP can potentially serve as a predictor of CVD risk in both MDS and non-MDS individuals.
Conclusion To our knowledge this is the first study in LR-MDS patients assessing longitudinally all critical parameters and providing objective measurements of subclinical atherosclerosis in all vascular beds. Our preliminary results indicate that the established CVD prediction models do not operate properly in LR-MDS patients and reinforce the notion of a pro-atherogenic role of MDS. Accurate assessment of CVD risk and prompt detection of subclinical cardiovascular disease in MDS patients will assist clinical decisions on the introduction of intensive monitoring and preventive use of CVD-specific interventions in these individuals.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal